 Sabtu, 31 Mei 2025 (03:05)
Sabtu, 31 Mei 2025 (03:05)
 Music |
 Video |
 Movies |
 Chart |
 Show |
 |
The ionization constant of nitrous acid is \( 4.5 \times 10^{-4} \)... (PW Solutions) View |
 |
The ionization constant of nitrous acid is 4.5 × 10^-4. Calculate the pH of 0.04 M sodium nitrite... (PW Solutions) View |
 |
The ionization constant of nitrous acid is `4.5xx10^(-4)`. Calculate the `pH` of `0.04 M` sodium (Doubtnut) View |
 |
The ionization constant of nitrous acid is `4.5xx10^(-4)`. Calculate the `pH` of `0.04 M` sodium... (Doubtnut) View |
 |
Ionic equilibrium / Q. 61 / CBSE board / class 11 (Chemistry Gyan Academy) View |
![Download Lagu Chemistry - Acids u0026 Bases (27 of 45) How to Find the [H+] and pH of Nitrous Oxide Thumbnail](https://img.youtube.com/vi/bw-avAN_3Cs/mqdefault.jpg) |
Chemistry - Acids u0026 Bases (27 of 45) How to Find the [H+] and pH of Nitrous Oxide (Michel van Biezen) View |
 |
Equilibrium Exercise Question 61: Class XI/NEET/IIT (LiveTutelage) View |
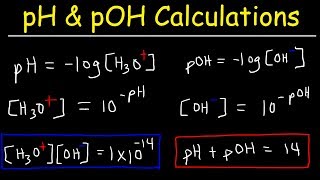 |
pH, pOH, H3O+, OH-, Kw, Ka, Kb, pKa, and pKb Basic Calculations -Acids and Bases Chemistry Problems (The Organic Chemistry Tutor) View |
 |
Nitrite / nitrous acid buffer problem (lseinjr1) View |
 |
Calculate the concentration of HF (manasditya publications) View |